Abstract Introduction: With the 2010 Nobel Prize in Physics awarded to the inventor of graphene, two British physicists, André and Constantine, set off a research boom in graphene for a time. Graphene has also become the material of choice for more and more scientists. same with...
Introduction: With the 2010 Nobel Prize in Physics awarded to the inventor of graphene, two British physicists, André and Constantine, set off a research boom in graphene for a time. Graphene has also become the material of choice for more and more scientists. Also in 2010, researchers at the Institute of Chemistry of the Chinese Academy of Sciences invented a new member of the carbon family: Graphene, which is an exciting breakthrough. People can't help but ask how the graphene acetylene has developed recently. What are the advantages and disadvantages of graphene and graphene? Who can dominate the future of "foreign goods" and "national goods"? And listen to the small series. New member of the carbon family
The synthesis and separation of new different-dimensional carbon allotropes have been the focus of research in the past two or three decades. Scientists have discovered new carbon allotropes such as three-dimensional fullerenes, one-dimensional carbon nanotubes and two-dimensional graphene. These materials have become the frontiers and hotspots of international academic research. Carbon materials can be widely used in lithium ion batteries, supercapacitors, sensors, solar cells, catalytic carriers, and nanoelectronic devices. Carbon has three hybrid states of sp3, sp2 and sp. Different heterogeneous states can form allomorphs of various carbons, such as: diamond can be formed by sp3 hybridization, and carbon nanoparticle can be formed by sp3 and sp2 hybridization. Tubes, fullerenes, graphenes, and the like. In 1996, the Chemical Nobel Prize was awarded to the discoverers of three fullerenes. In 2010, Andrei Heim and Konstantin Novoslov of the University of Manchester in the United Kingdom were pioneering in the two-dimensional graphene. The research was awarded the Nobel Prize in Physics, which brought the research of carbon materials to a new stage, and also ignited the enthusiasm and interest of scientists in the study of allotropes of new carbon.
Since the carbon-carbon triple bond formed by the sp hybridization state has the advantages of linear structure, no cis-trans isomer and high conjugate, it has been desired to obtain a new allotrope of carbon having a sp hybrid state, and it is considered that Carbon-based materials with excellent electrical, optical and optoelectronic properties will be the key materials for the next generation of new electronic and optoelectronic devices. At this time, our scientists have tried to witness the birth of a new member of the carbon family, graphene, for the first time in the world.
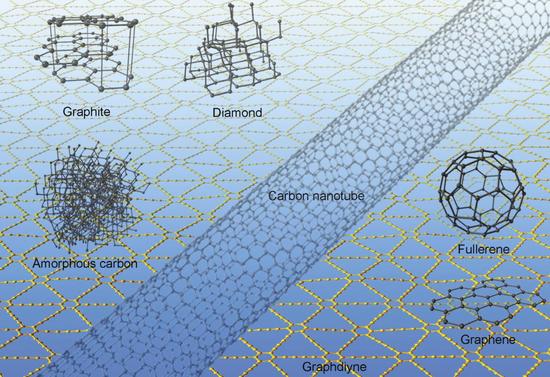
Carbon family family portrait
Straw is still gold? In 2010, when the graphene won the Nobel Prize in Physics, our scientists were in Chem. Commun. The magazine first reported the birth of a new member of the carbon family, graphene.
For a time, the world has not slowed down. What is graphene? I don’t understand, what kind of ghost is graphene? Some people with more brain openings are already looking forward to graphene! Just as the first-line stars have many imitators, the celebrity effect is mixed with the rivers and lakes. People think that the graphite alkyne is famous for its aura of graphene. Under the prestigious name, it is actually difficult.
But is this really the case?
When people hold the mentality of trying to understand the graphite alkyne, they suddenly feel shocked, this is really a baby, it is really late to meet each other!
In 2010, researchers from the Key Laboratory of Chemical Solids Institute of the Chinese Academy of Sciences used the coupling reaction of hexadecanylbenzene under the catalysis of copper sheets to successfully synthesize new areas of large-area carbon on the surface of copper sheets by chemical methods. Alien - Graphene, which is the first large-area graphite alkyne film in the world. It has abundant carbon chemical bonds, large conjugated system, wide interplanar spacing, and excellent chemical stability. It is known as the most stable allotrope of synthetic diacetylene. Graphene alkyne can be widely used in electronics, semiconductors and new energy fields due to its special electronic structure and excellent semiconductor properties similar to silicon.
It has long been desired to obtain new allotropes of carbon having sp hybridized state, thereby obtaining excellent performance. Such a good thing, why can't foreigners do it, and Chinese scientists have made it?
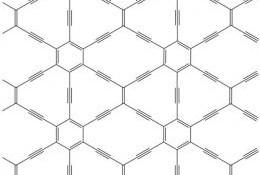
Molecular structure of graphene
Beautiful "accident" In 1968, the famous theoretician Baughman realized that the graphene alkyne structure can exist stably. The internationally renowned functional molecules and polymer research groups have started related research, but they have not been successful.
Gradually, people began to wonder if graphene can be artificially synthesized.
Until 2010, Li Yuliang, a researcher at the Institute of Chemistry of the Chinese Academy of Sciences, proposed a new allotrope-graphene alkyne that successfully obtained a large area (3.62 cm2) of carbon by chemically in situ synthesis of graphene on the surface of copper. Graphdiyne) film. In this process, the copper foil not only serves as a catalyst for the cross-coupling reaction, but also serves as a growth substrate, and provides a large planar substrate for the directional polymerization required for the growth of the graphene film.
By visiting Li Yuliang, we learned that the huge "accident" of graphene is actually the accumulation of experience of the research team for many years.
Li Yuliang's research group began research on the molecular design of the source, and gradually tried to synthesize fragments of some molecules. But just quantitative change is not enough, until one day unexpected burst of inspiration - in the process of reading the literature, Li Yuliang researchers suddenly thought of a chemical method that could make a large area of ​​graphite alkyne film. So they immediately proceeded to do it, the qualitative change happened, and the world was shocked!
"Super Ability" of "Super Materials"
As early as 1968, former Soviet physicists proposed the "Fisslag theory" and predicted fictional materials with peculiar properties, which have extraordinary physical properties not possessed by natural materials, such artificial composite structures or composite materials. It is "super material." The super-capacity of super materials comes from the novel design ideas of scientists. The design idea of ​​super materials indicates that people can artificially acquire “new substances†with extraordinary physical properties that are quite different from those in nature without prejudice to the basic laws of physics, bringing the design and development of functional materials into the environment. A brand new world.
It is precisely because of the super-capacity of super materials that the new material field has set off a technical frenzy, and all countries have actively joined the research and development of “super materialsâ€. The battle for "super power" is getting worse and worse. Whose super power is more eye-catching? Can those bridges that sound so far as sci-fi movies really go out of the lab and enter people's lives? It is precisely because of the expectations and uncertainties of the future world that the battle for super materials is bound to be an unknown.
Chess opponent: graphene VS graphite alkyne
As a newcomer to the carbon family, graphene has become synonymous with “magical material†since its birth. The top scientific research powers of various countries have become the leader in its material industry. However, the appearance of graphene has once again refreshed the heat of the new word "graphene". The two are playing against each other, so who is better?
Let me talk about the extraordinary aspects of graphene.
Graphene is both the thinnest material and the toughest material, with a breaking strength 200 times higher than the best steel. At the same time, it has good elasticity and can reach 20% of its own size. It is currently the thinnest and strongest material in nature. If a 1 mm square graphene is used as a hammock, it can weigh one kilogram of cat by weighing less than 1 milligram.
The most promising application of graphene is to become a substitute for silicon, manufacturing ultra-micro transistors for the production of future supercomputers. By replacing silicon with graphene, computer processors can run hundreds of times faster.
In addition, graphene is almost completely transparent, absorbing only 2.3% of light.
At the same time, it is very dense, even the smallest gas atoms (deuterium atoms) can not penetrate. These features make it ideal for use as a raw material for transparent electronic products such as transparent touch displays, luminescent panels and solar panels.
As a new type of nanomaterial that is currently found to be the thinnest, strongest, and most conductive and thermally conductive, graphene is called "black gold" and is the "king of new materials." Scientists even predict that graphene will "completely change the 21st century." "It is very possible to set off a revolutionary new technology and new industrial revolution that has swept the world."
Graphene alkyne is a new all-carbon nanostructured material after fullerenes, carbon nanotubes and graphene. It is a novel allotrope of carbon formed by the hybridization of sp and sp2. It is a full carbon material with a two-dimensional planar network structure formed by conjugated benzene rings by 1,3-diyne bonds. The carbon chemical bond, large conjugated system, wide-face spacing, and excellent chemical stability are known as the most stable allo-forms of synthetic diacetylene.
Due to its special electronic structure and excellent semiconductor properties similar to silicon, graphene is expected to be widely used in electronics, semiconductors and new energy fields.
Recently, the research team led by Huang Changshui, a researcher at the Energy Application Technology Branch of the Qingdao Institute of Bioenergy and Processes, Chinese Academy of Sciences, and Li Yuliang, a researcher at the Institute of Chemistry of the Chinese Academy of Sciences, first applied graphene to electrode materials for lithium-ion batteries and electrochemically stored lithium. The performance and lithium storage mechanism were analyzed in detail, and the structure-activity relationship between the structure, morphology and electrochemical properties of graphene was elucidated. The application of graphene materials in lithium batteries was explored.
These studies provide theoretical basis and experimental guidance for the study of lithium storage properties of the graphene family and the exploration of new carbon energy storage materials. Studies have shown that graphene is an ideal lithium storage material, and its unique structure is more conducive to the diffusion and transmission of lithium ions in and out of the plane, which gives it a very good rate performance, from the practice proved that graphite alkyne It is a very promising lithium storage energy material, and scientists also predict that it will have an extraordinary impact in the new energy field.
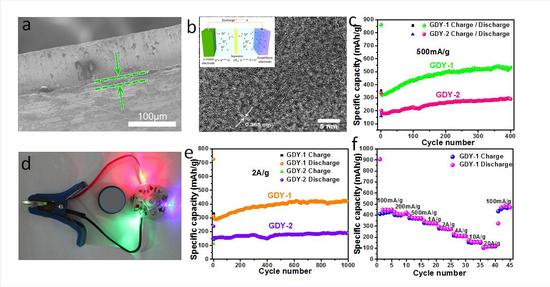
Graphene alkyne for lithium ion battery electrode materials
   From this point of view, in terms of performance and application prospects, the "super ability" of graphene is not inferior to graphene. As a new material material that has been the cusp of the scientific community, the results of graphene and alkyne from China are naturally not bad. The battle for "super materials" at home and abroad has just begun, and the confrontation between graphene and graphene is continuing, but we have reason to believe that Chinese scientists are bound to take another step in this master's trick. Marble Pattern Steel Coil,Colored Steel Sheet Coil,Marble Metal Sheet,Marble Color Coated Steel
Shandong Wofeng New Material Co., Ltd. , https://www.wofengcoil.com