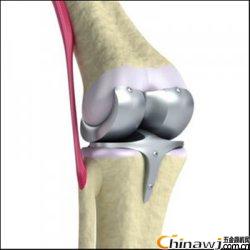
For total knee prostheses, the FDA recommends a reference to the ASTM F1800 porous coated metal neck fatigue test. The FDA recommends testing five samples under different loads to develop a force/quantity (AF/N) cycle curve containing at least one sample of 10 million cycles.
For total knee prostheses, the FDA recommends a reference to the ASTM F1800 porous coated metal neck fatigue test.
 Editor: (Hardware Business Network Information Center) http://news.chinawj.com.cn
Editor: (Hardware Business Network Information Center) http://news.chinawj.com.cn 
307 Bore Type Thread Insert,Threaded Hole Insert 307 Self Tapping,307 Type Self-Tapping Thread Insert,307 Thread Insert
Shenyang Helisert Technology Co., Ltd , https://www.helisert.com